Compared to oral and transdermal medicine, parenteral formulations are potent and fast-acting. With that comes a special set of considerations. In this article, we'll cover key ingredients, delivery methods, and manufacturing factors. Whether you’re working on a novel, generic, or adapted drug, these are all things you’ll need to know to develop a successful formulation.
Key components in parenteral formulations
Parenteral formulations can be developed in several different formats. The most common is called a solution, in which the active ingredient is dissolved in a liquid. Another common format is called a suspension. If the active ingredient is insoluble, a suspension allows the ingredient to be suspended in a liquid instead.
While each formulation is different, they do share the same basic structure. Understanding their key components will help you grasp what’s required to create a successful parenteral drug.
 Active Pharmaceutical Ingredient (API)
Active Pharmaceutical Ingredient (API)
Think Acetaminophen in Advil®. The Active Pharmaceutical Ingredient (API) is the substance responsible for the therapeutic effect. It’s the cornerstone of the formulation.
Solvents
This is the liquid in which the API is dissolved or suspended. Common solvents include water, saline, alcohol, or glycerin. They ensure that the medication is delivered in a form that the body can absorb.
Antioxidants
Antioxidants are added to prevent the oxidation of the Active Pharmaceutical Ingredient (API). This helps maintain the stability of the formulation over time. Common antioxidants are ascorbic acid and tocopherols.
Buffers
By keeping the pH within a specific range, buffers help ensure that the API remains stable and is well-tolerated by the body. Examples of common buffers are sodium phosphate and citrate.
Preservatives
Preservatives are essential in preventing microbial growth, especially in multi-dose vials. Without preservatives, the formulation could pose significant health risks to patients. Common preservatives include benzyl alcohol, phenol, and parabens.
Chelating agents
Chelating agents such as EDTA (ethylenediaminetetraacetic acid) help stabilize the formulation by sequestering unwanted ions, preventing them from interacting with the API or other components.
Viscosity modifiers
Proper viscosity is crucial for ensuring smooth and consistent administration of the drug. Modifiers can make the solution thicker or thinner, depending on the desired injection properties. Common viscosity modifiers include substances like polyethylene glycol (PEG) and carboxymethyl cellulose (CMC).
Isotonicity agents
Isotonicity agents help prevent pain and tissue damage upon injection. Without this, the injection can cause cells to shrink or swell, leading to discomfort or injury. Common isotonicity agents include sodium chloride and dextrose.
Transporters
Finally, transporters, also known as carriers, may be included to ensure the drug gets where it needs to be in the body. While some formulations are meant to be localized, others are meant to travel through the entire body.
The bulk of the formulation development process involves finding what combination of these ingredients will result in a potent, reliable recipe.
Injectable delivery methods for parenteral drugs
While all parenteral drugs follow the same basic principles, they can be administered in many different ways — a key consideration when it comes to formulation. Each type of injectable has its specific benefits and is chosen based on the required speed of action, targeted delivery site, and overall goals.
Intramuscular (IM) injections
Intramuscular injections are delivered directly into a muscle. This method is commonly used for vaccines and medications that require quick absorption into the bloodstream.
Subcutaneous injections
Subcutaneous injections are administered just under the skin. This delivery method is often used for drugs that require a slower, steady absorption into the bloodstream, such as insulin.
Intravenous (IV) injections
Intravenous injections are delivered directly into a vein, offering immediate access to the bloodstream. This method is ideal for rapid drug delivery, often used in emergency situations or for medications that need to work instantly.
Intrathecal injections
Intrathecal injections involve delivering medication into the spinal canal. They are typically used for pain management or administering anesthesia during surgery, providing direct access to the central nervous system.
Intraperitoneal injections
Intraperitoneal injections are delivered into the peritoneal cavity, the space surrounding the abdominal organs. This method is used for treatments such as chemotherapy, allowing for efficient absorption of the medication by the abdominal organs.
Considerations for manufacturing parenteral medication
When it comes to manufacturing parenteral drugs, there are several key considerations to ensure the safety, efficacy, and quality of the final product.
Aseptic fill-finish
Because injectable drugs bypass the body’s primary protective systems (the skin and mucous membranes), they must be manufactured in a sterile environment. All equipment, containers, and closures used in the process must be sterilized before use. Personnel involved in the process must adhere to strict guidelines and wear protective clothing.
Regulatory compliance
With increased attention to sterility comes increased regulatory scrutiny. Not only must the manufacturers follow sterile practices but they must also document their efforts for the FDA. Additional testing is required for parenteral formulation to ensure that the drug is free from particulate matter, pyrogens, and other contaminants.
Scalability
Just because a parenteral formulation is promising in small batches doesn't mean it’s a guarantee at the commercial level. Chemical reactions may behave differently in larger batches due to changes in surface area-to-volume ratios, affecting reaction rates and potentially leading to incomplete reactions or by-product formation. Part of the formulation development process involves working through those possibilities and adjusting the formulation until it’s proven to scale in a reliable way.

Talk to a parenteral formulation expert
Developing parenteral formulations is a complex process. But the demand is high, and the outcome can be very rewarding. With an understanding of key ingredients, delivery methods, and manufacturing, you have a solid foundation for developing a successful formulation.
Have a parenteral project on the horizon? Talk to one of our formulation experts. American Injectables — unsurprisingly — specializes in injectable formulations. We can provide the equipment and know-how you need to bring your medicine to market.

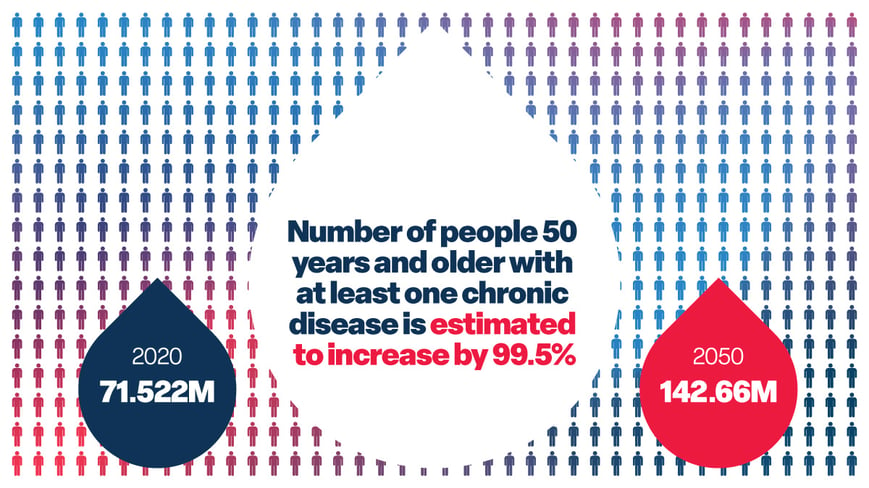

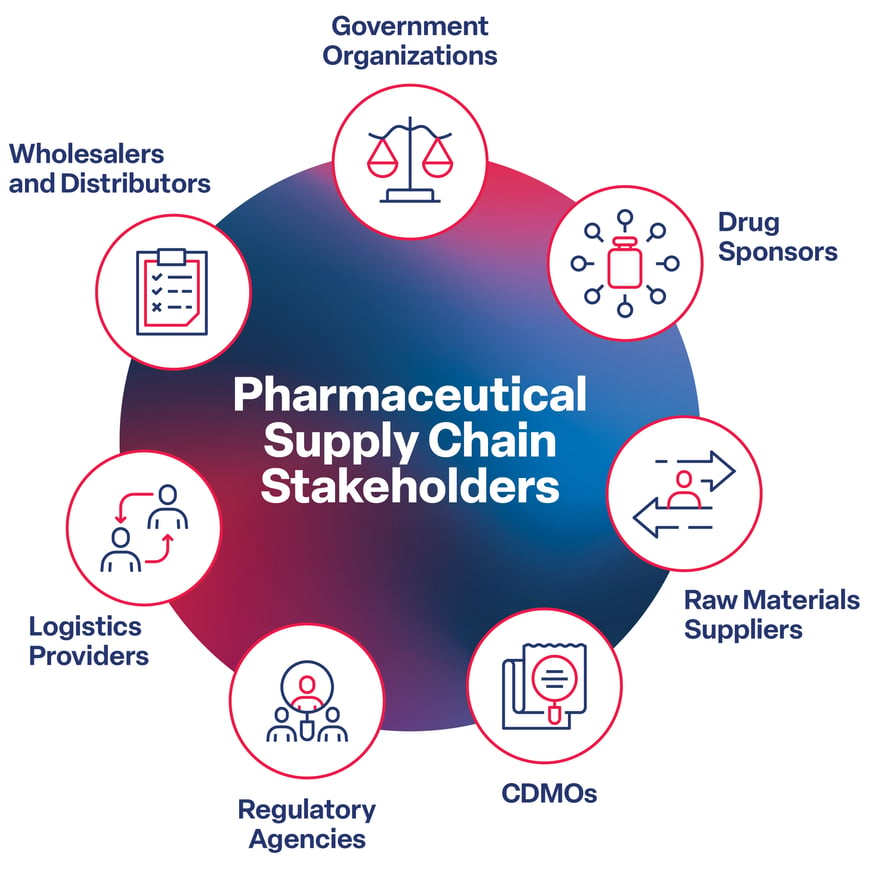
No Comments