American Injectables Earns FDA Approval
BROOKSVILLE, FL (January 9, 2025) — American Injectables, a contract development and manufacturing organization (CDMO) specializing in sterile injecta...
Read MoreMomentum and Milestones: 2025 in Review
Year End Reflections at American Injectables As we conclude the year, American Injectables stands at...
Manufacturing Commercial Success
Delivering Our First Commercial Success American Injectables recently completed commercial productio...
FDA’s ANDA Pilot: Win for U.S. Manufacturing
FDA’s ANDA Prioritization Pilot: What It Means for U.S. Generics and CDMOs
Digital Confidence: Building Trust in Sterile Manufacturing
The sterile injectable space is evolving quickly, and capacity and compliance alone are no longer en...
Pharma Tariffs Push for U.S. Production
What the New Pharma Tariff Means for CDMOs and Sterile Injectables The U.S. just announced a propose...
Bigger and Built to Deliver.
In sterile injectable manufacturing, growth isn’t optional, it’s key.
Key Leadership Appointments to Drive Future Growth
American Injectables, a leading contract development and manufacturing organization (CDMO) specializ...
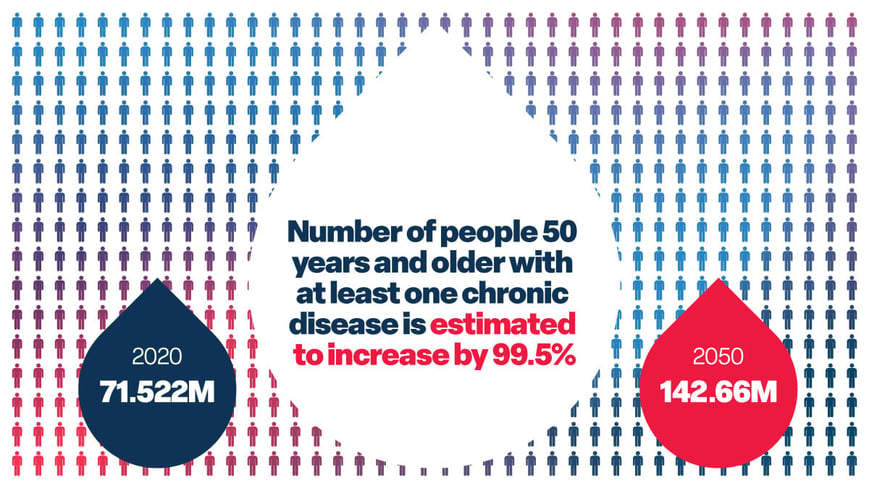
A Business-Minded Guide to Injectable Solutions and Market Trends
You don’t need a PhD in biochemistry to work in the pharmaceutical industry. However, understanding ...
Pharmaceutical Data Integrity: Protecting Your Branded Formulation
Imagine spending years and hundreds of thousands of dollars on a formulation, only to have it compro...