Momentum and Milestones: 2025 in Review
Year End Reflections at American Injectables As we conclude the year, American Injectables stands at a pivotal point in its evolution. What began as a...
Read MoreFDA’s ANDA Pilot: Win for U.S. Manufacturing
FDA’s ANDA Prioritization Pilot: What It Means for U.S. Generics and CDMOs
Digital Confidence: Building Trust in Sterile Manufacturing
The sterile injectable space is evolving quickly, and capacity and compliance alone are no longer en...
Pharma Tariffs Push for U.S. Production
What the New Pharma Tariff Means for CDMOs and Sterile Injectables The U.S. just announced a propose...
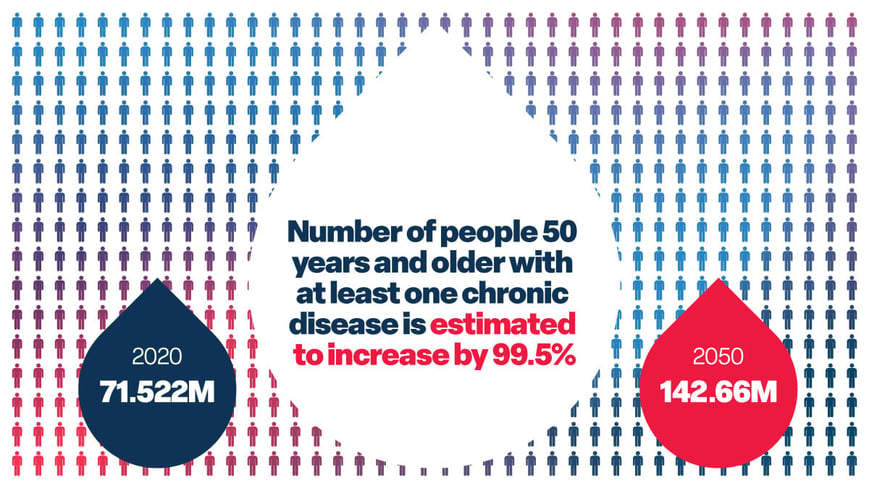
A Business-Minded Guide to Injectable Solutions and Market Trends
You don’t need a PhD in biochemistry to work in the pharmaceutical industry. However, understanding ...
Special Considerations for Developing Parenteral Formulations
Compared to oral and transdermal medicine, parenteral formulations are potent and fast-acting. With ...