Momentum and Milestones: 2025 in Review
Year End Reflections at American Injectables As we conclude the year, American Injectables stands at a pivotal point in its evolution. What began as a...
Read MoreManufacturing Commercial Success
Delivering Our First Commercial Success American Injectables recently completed commercial productio...
FDA’s ANDA Pilot: Win for U.S. Manufacturing
FDA’s ANDA Prioritization Pilot: What It Means for U.S. Generics and CDMOs
Digital Confidence: Building Trust in Sterile Manufacturing
The sterile injectable space is evolving quickly, and capacity and compliance alone are no longer en...
Pharma Tariffs Push for U.S. Production
What the New Pharma Tariff Means for CDMOs and Sterile Injectables The U.S. just announced a propose...
Key Leadership Appointments to Drive Future Growth
American Injectables, a leading contract development and manufacturing organization (CDMO) specializ...
Special Considerations for Developing Parenteral Formulations
Compared to oral and transdermal medicine, parenteral formulations are potent and fast-acting. With ...
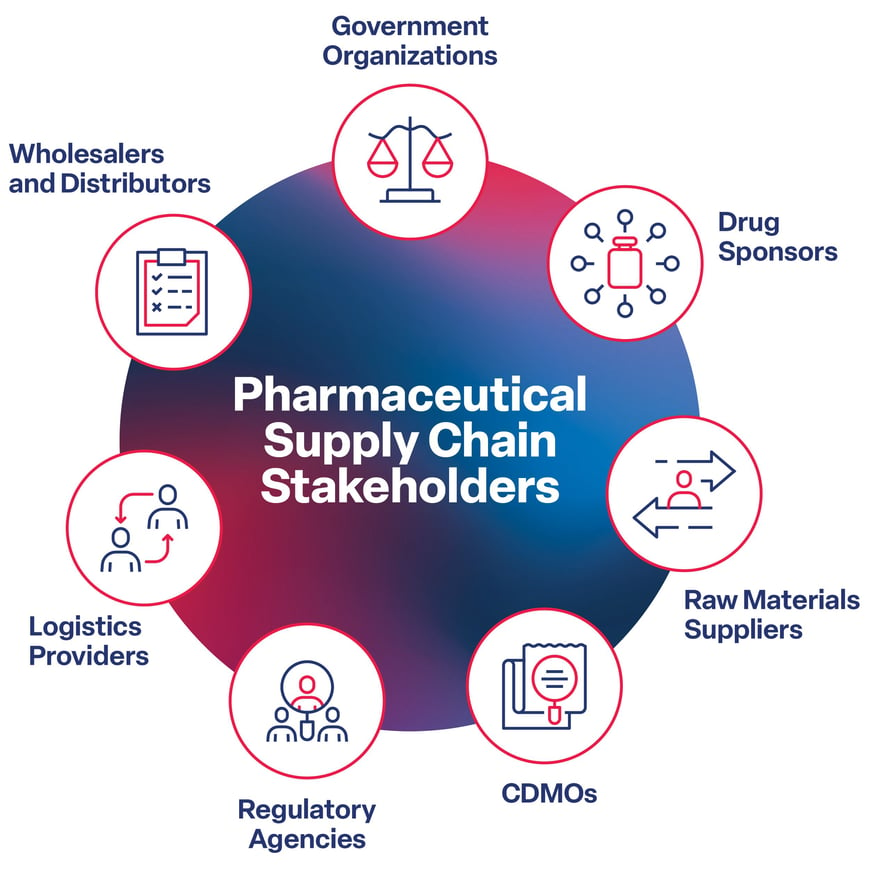
Strengthening the Pharmaceutical Supply Chain: It Starts With Formulation
The effects of supply chain issues are often most noticeable at the consumer level, manifesting as p...
Developing a Regulatory Strategy: Key Studies in the Formulation Stage
The FDA approval process is time-intensive and difficult for good reason. If you emerge on the other...
Formulation Development Process for Injectables: Your Steps to Success [Graphic]
Formulation development can take as long as three U.S. presidential terms. With that kind of time, i...